
The Science of Snowflakes: Exploring the Formation of Winter’s Frozen Wonders
When snow blankets The Parklands, it transforms the landscape into a sparkling winter wonderland. But have you ever stopped to think about the science behind the many delicate flakes that form this snow cover? Snowflakes are more than just frozen water—they are complex formations shaped by the forces of nature.
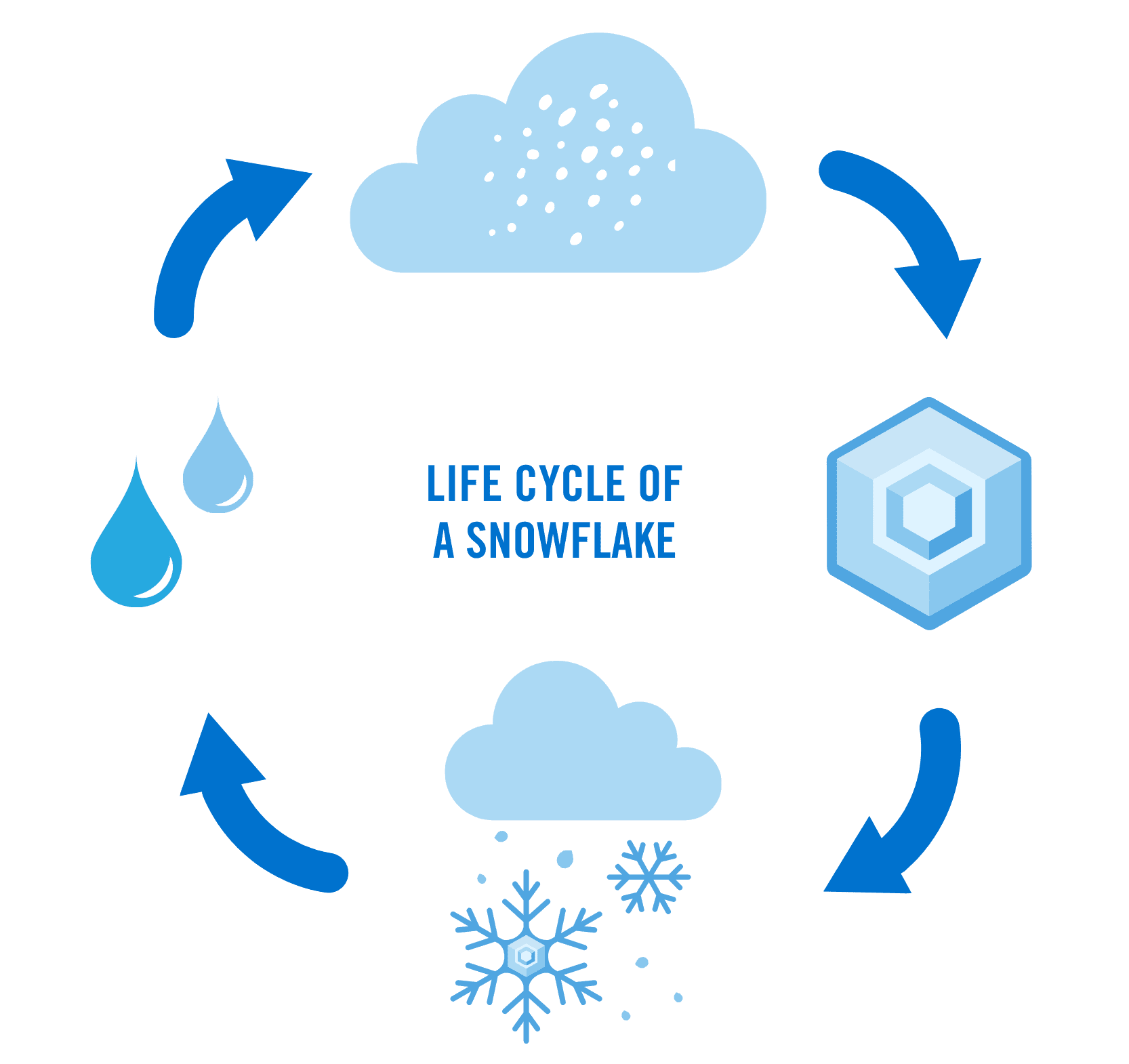
The Life Cycle of a Snowflake
The journey of a snowflake begins high in the atmosphere, where cold temperatures and tiny particles like dust or pollen set the stage for its formation. Here’s how it happens:
- Nucleation
A snowflake begins its journey high in the atmosphere as a tiny particle, often dust or pollen, that acts as a seed for ice crystals. When the temperature in the atmosphere is below freezing, water vapor condenses and freezes around this particle and the crystal begins to grow. This is the “birth” of the snowflake. - Growth
As the ice crystal falls, it collects more water vapor, which freezes onto its surface. This growth process is influenced by temperature and humidity, shaping the snowflake into various forms—from geometric hexagonal plates to intricate stellar dendrites. (Keep reading for more info on types of snowflakes!) - Journey to the Ground
The journey of the snowflake can take anywhere from a few minutes to several hours. As the snowflake continues its descent, it can encounter fluctuating atmospheric conditions. These changes in temperatures and humidity can affect its size, shape, and even lead to melting, refreezing, and/or collisions with other snowflakes. - Resting Place
Once a snowflake lands, its journey isn’t over. Snow on the ground begins to change immediately, undergoing a process called metamorphism. Wind, sunlight, and temperature fluctuations reshape the crystals, turning fluffy snow into compact ice or melting it back into water. This life cycle affects how snow interacts with the environment, from insulating plant roots to providing habitats for animals.
Types of Snowflakes
If you look closely at falling snow, you can see many different crystal shapes! How each snowflake gets its unique shape depends on the temperature and humidity of the air as it drifts to the ground. Warm, humid air can create snowflakes with intricate branches and patterns while cold, dry air can produce snowflakes with simpler designs. Snowflakes come in a variety of shapes, including:
- Plates: The basic shape of a snow crystal is a hexagonal prism, due to the molecular structure of water which forms hexagonal patterns when it freezes. Depending on how fast each side of the hexagon grows, it can turn into flat plates, including hexagonal plates (simple, flat, six-sided crystals), stellar plates (thin, star-shaped crystals), and sectored plates (similar to stellar plates, but with more prominent ridges between prism facets). Occasionally, plates can form truncated shapes known as triangular crystals, with only three sides.

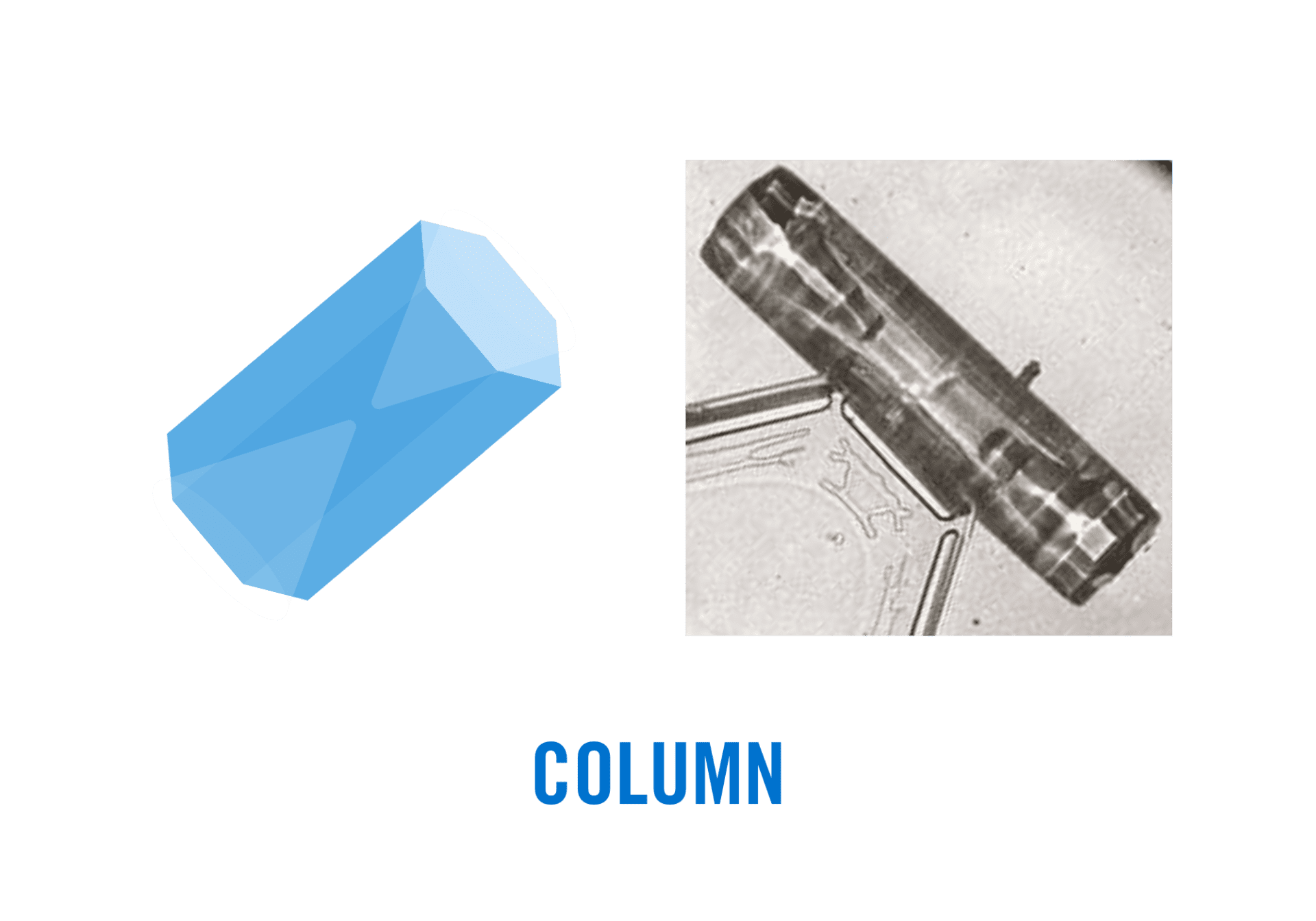
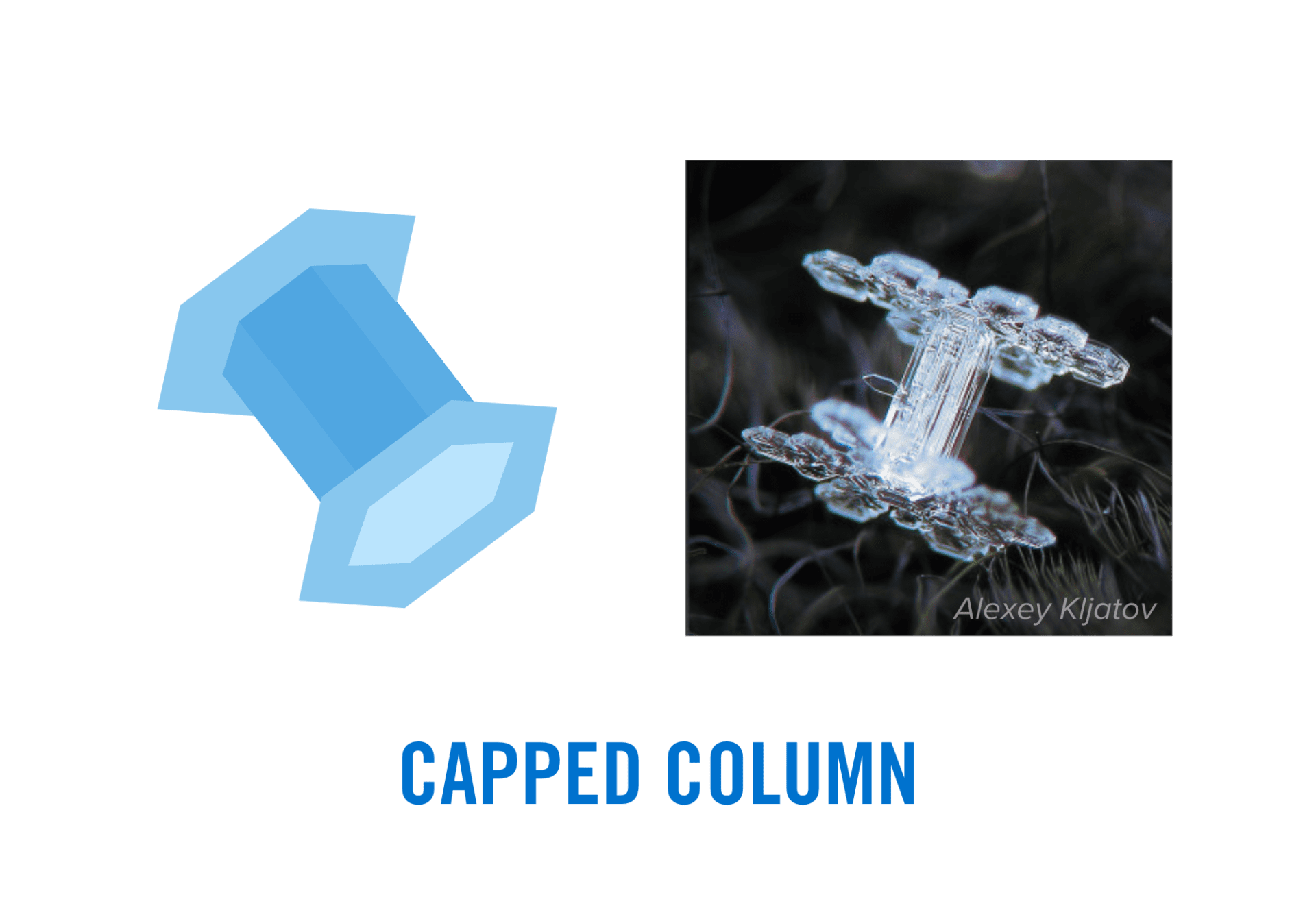
- Columns: Columns are cylindrical snowflakes that are quite small and shaped similarly to a wooden pencil. These snowflakes appear in moderately cold temperatures and often form with conical hollow regions in their ends, which are known as hollow columns. However, some short and stubby column crystals can blow into an area where the growth becomes more plate-like. In this case, two flat structures grow at the ends of the ice column. This shape is called a capped column or, if the center column is very short, a double plate.
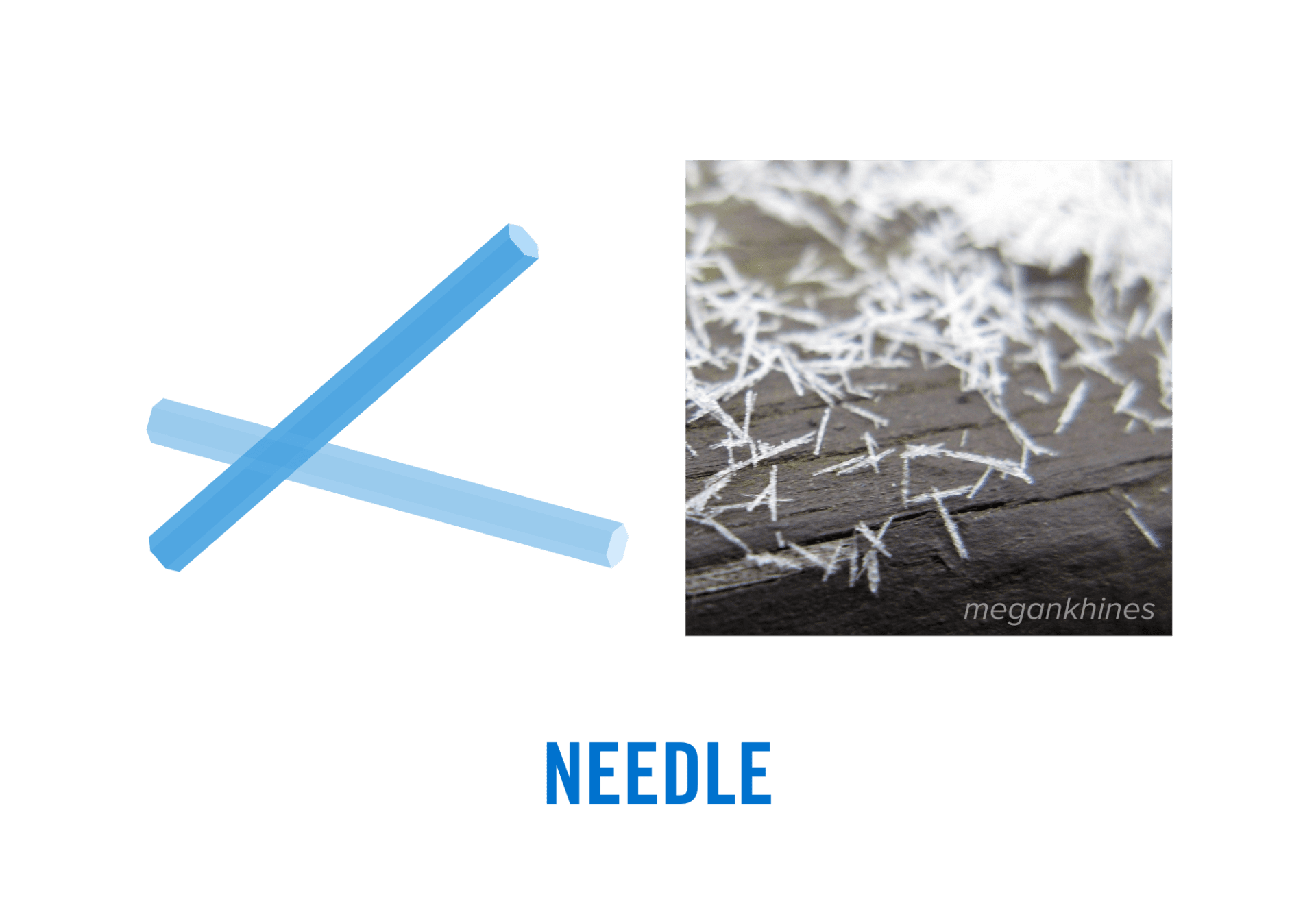
- Needles: The magical thing about snow crystals is that their shape can change dramatically from a flat plate to a long, slender needle when the temperature changes even by just a few degrees! When temperatures are very cold, you might be able to spot needle-like crystals. These columnar ice crystals are long and thin and can resemble small pieces of white hair.
- Stars and Dendrites: When a snowflake grows, it can become more complex and intricate in shape. “Dendrite” is derived from the Greek word dendron (meaning tree), so this name refers to crystals that are shaped like trees, complete with branch-like structures. These are usually larger than the simpler plate-shaped crystals. Stellar dendrites and fernlike stellar dendrites are star-shaped with delicate branches that are formed in colder, humid conditions. These types of crystals create the best “powder” snow, since they are so thin and light and therefore make less dense snowpack.

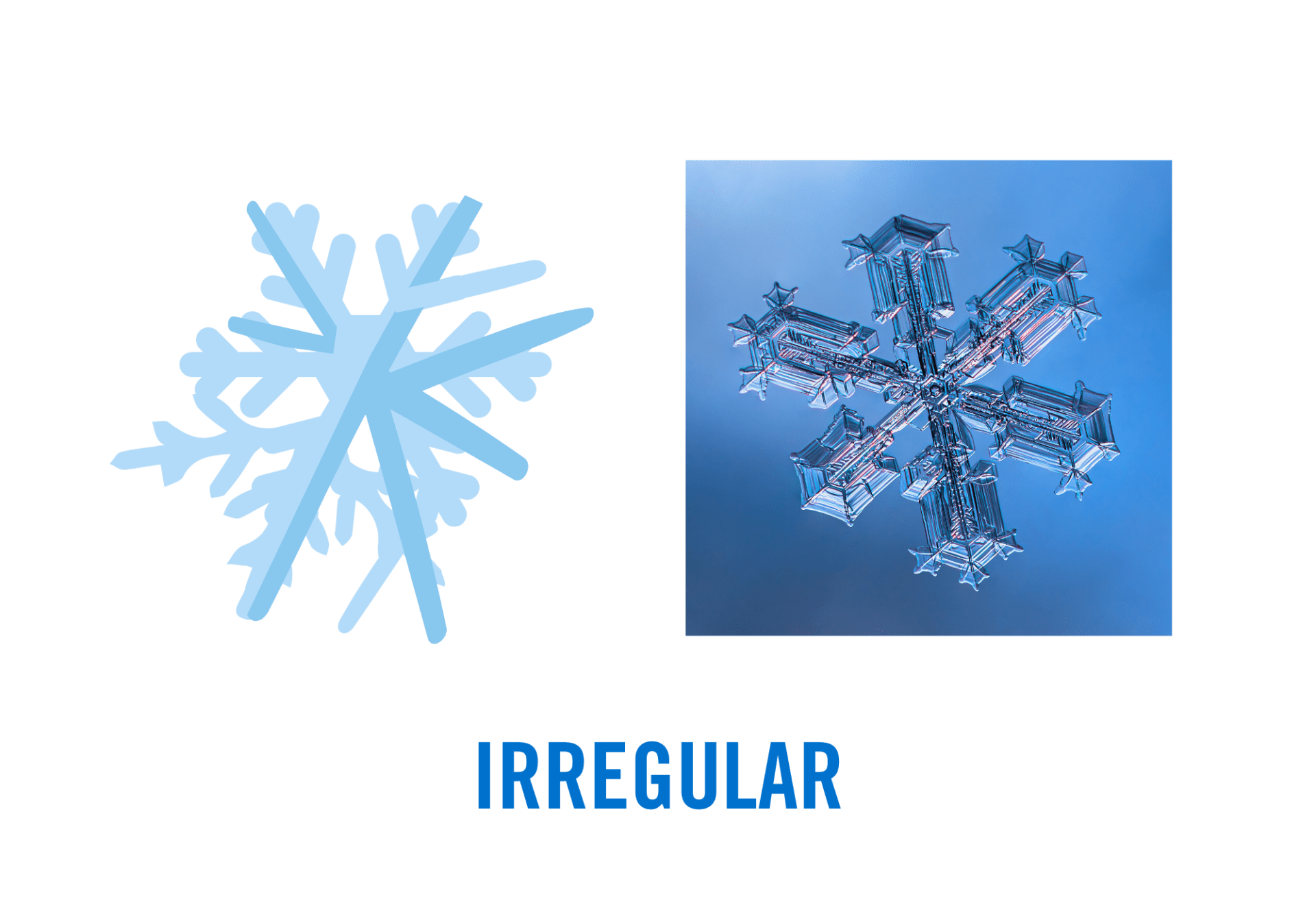
- Irregular Snowflakes: These are the most common type of snowflake, nonuniform and misshapen in appearance, resulting from collisions or environmental changes during descent. These are usually small and clumped together with little symmetry. Irregular snowflakes include damaged or partially melted snowflakes, snowflakes that have stuck together and refrozen, or otherwise misshapen crystals. Irregular snowflakes can also take on some specific forms, like when multiple crystals grow clumped together at random orientations (bullet rosettes), errant branches grow off the main crystal plane (radiating dendrites), or water droplets collide and stick to snow crystals forming rimed, snowball-like crystals like graupel.
These are some of the basic types of snowflake anatomy, but classifying snowflakes isn’t as straightforward as it might seem! The way snowflakes are grouped into types often depends on personal or scientific preference. A good comparison is with dog breeds—while specific breeds have been established by committees, one could theoretically create countless classifications. Just like some mixed-breed dogs don’t fit neatly into one category, some snowflakes don’t align perfectly with any one type. Snowflakes do vary in shape and structure, and naming these types is helpful for discussion, but scientists have developed many different classification systems.
Activity: Create Your Own Snowflakes
You don’t need a snowy day to explore the beauty of snowflakes! Want to bring some of that winter magic indoors? Here’s a fun project to try at home, where you can create your own sparkling “snowflakes” using borax.
Borax Snowflakes
Materials:
- Borax (found in the laundry aisle)
- Pipe cleaners
- String
- A pencil or chopstick
- A heat-safe jar or glass
- Boiling water
- Watercolor paint (optional)
Instructions:
- Twist the pipe cleaners together to form a snowflake shape. (1-2 pipe cleaners per snowflake should do the trick.)
- Tie one end of a piece of string to the snowflake and the other to the pencil.
- Fill the jar with boiling water and stir in Borax until it dissolves (about 3 tablespoons of Borax per cup of water).
- Suspend the snowflake in the solution, ensuring it doesn’t touch the sides.
- Leave it overnight. By morning, your snowflake will be covered in sparkling crystals! You can leave your snowflake in the solution until your snowflake reaches your desired level of crystallization.
- For some extra customization, you can lightly brush your snowflake with watercolor paints and watch as the paint absorbs into the crystal.
Note: This project involves boiling water and Borax, which can be a mild irritant. Adult supervision is highly recommended, especially when handling hot liquids. Avoid ingesting the solution. Store materials out of reach of children and pets.
Check out Go Science Kids for more information about this DIY science project, including a comparison of other methods for coloring the snowflakes!

